Options
Mass Spectrometer (MS)
Multicomponent adsorption studies often require a mass spectrometer (MS) to monitor the residual gas composition. The MS is the most common detector system used for breakthrough analysis.
FTIR Analyzer (FTIR)
FTIR spectrometers are often selected for experimental breakthrough studies such as the separation of xylenes or other aromatic hydrocarbons.
Humidity Sensor
Allows direct tracking of water content for low cost. Can be useful especially in production control applications.
Sample Preperation System
Small quantities of active material can be mixed with an inert carrier to produce a homogeneous sample and improve analysis reproducibility.
CO2 Sensor
Allows direct tracking of CO2 content for low cost. Can be useful especially in production control applications.
Sample Column (Different Volume)
The BTA may be used with a variety of column diameters to accommodate different sample morphologies included powders, pellets, and extrudates.
MFC and Mixing Valves (Maximum of 6 Gas Inlets)
Additional mass flow controllers and blending valves may be added to the BTA to increase the analytical capabilities and expand the range of experiments that may be conducted.
Vapor Source (Max of 2)
Moisture or other vapors such as xylenes or other aromatics are compatible with the optional vapor sources available for the BTA.
High Pressure Adsorption
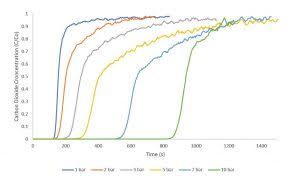
Zeolite 13X has been extensively studied for applications in catalysis and adsorption. In this study, zeolite 13X was used as an adsorbent for carbon dioxide adsorption to collect breakthrough curves from 1 – 10 bar pressure. These measurements were collected using equimolar flow rates of 10 sccm nitrogen and 10 sccm carbon dioxide. A 1 sccm stream of helium was used as a tracer gas to determine the start of the breakthrough experiment. All measurements were collected at an analysis temperature of 30°C. Between each measurement, the zeolite 13X sample was reactivated overnight to ensure complete desorption of carbon dioxide. The figure shows a consistent increase in breakthrough time across successive experiments as the pressure is increased.
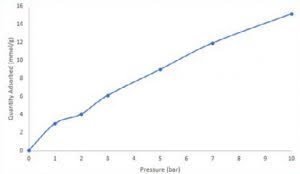
Following carbon dioxide breakthrough measurements an equilibrium adsorption quantity was calculated for each curve by solving the breakthrough equation. Next, an isotherm was constructed displaying the quantity of carbon dioxide adsorbed at 1, 2, 3, 5, 7, and 10 bar total pressure. At 10 bar, zeolite 13X adsorbed roughly 15 mmol/g carbon dioxide. While isothermal data collected via breakthrough cannot be directly correlated with static adsorption measurements, it can provide an assessment of an adsorbent in process-relevant conditions.
CO2 Adsorption
Single-component carbon dioxide breakthrough adsorption experiments were conducted on zeolites 13X and 5A, and metal-organic frameworks MIL-53(Al) and Fe-BTC. All materials were analyzed at 30°C while flowing an equimolar gas stream consisting of 10 sccm nitrogen and 10 sccm carbon dioxide. A 1 sccm stream of helium was also blended into the feed gas stream as a tracer gas to aid in identifying the start of the breakthrough experiment. The breakthrough curves for the four materials are plotted below on a mass-normalized axis. The total quantity of CO2 adsorbed follows the trend: molecular sieve 5A > zeolite 13X > Fe-BTC > MIL-53(Al). The table below shows the total quantity adsorbed in mmol/g.
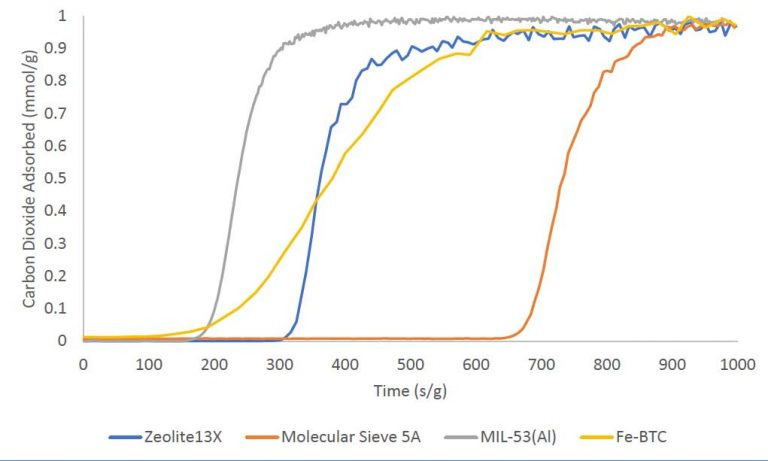
MATERIAL CARBON DIOXIDE ADSORBED(MMOL/G)
ZEOLITE 13X 2.94
MOLECULAR SIEVE 5A 3.52
MIL-53 (AI) 1.23
FE-BTC 2.30
Software
MicroActive the most intuitive, flexible, and comprehensive analysis software for adsorption studies
The flexible, intuitive, easy-to-use software allows for the widest range of experimental conditions and automates the breakthrough from sample activation to sample analysis, including the ability to perform cyclic experiments. Paired with industry leading MicroActive analysis software, the BTA system accurately and reproducibly characterizes adsorbents, analyzes data with comprehensive analysis methods, and solves the breakthrough equation for the most demanding samples.
MicroActive Software allows us:
Data reduction from MS
Quantity adsorbed selectivity
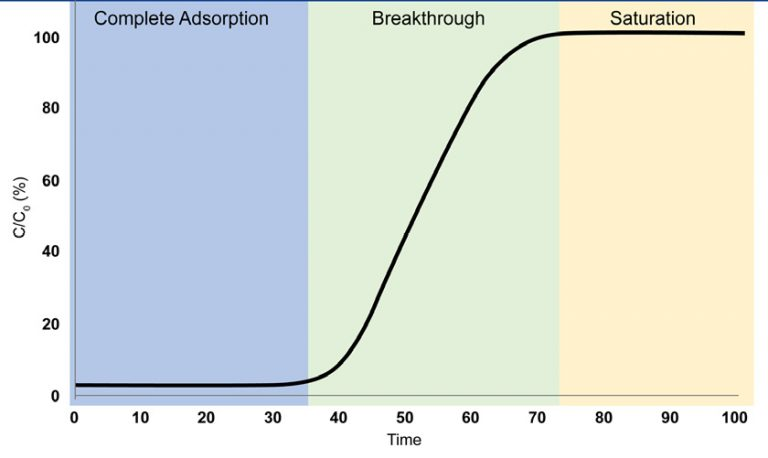
Examining Breakthrough Curve
Complete Adsorption
The adsorbate gas is completely adsorbed such that none can be detected at the outlet of the breakthrough column
Breakthrough
The adsorbate gas is first detected at the outlet of the breakthrough column. Gas continues to adsorb; however, the adsorbent is no longer able to adsorb the entirety of the gas that is entering the breakthrough column
Saturation
The adsorbent has reached saturation and can no longer adsorb the adsorbate gas, allowing it to pass through the column freely